Flowserve Valves in Pharmaceutical API Production
The active pharmaceutical ingredient industry manufactures APIs (active pharmaceutical ingredients) from raw materials through both chemical and physical means. Depending on the complexity of the molecule required, synthesis of APIs (or bulk pharmaceuticals) might require multi-step complex chemistry using a range of processing technologies, with the need for specialized valves for nearly every process step.
Regardless of where the active pharmaceutical ingredient is made, companies must adhere to strict safety and quality standards set by the country where it will be used, such as the FDA or the European Medicines Agency. It’s important that API manufacturers can keep production online with valve products like Flowserve’s that are designed for the needs of the pharmaceutical industry.
Advantages of Flowserve Valves for the Pharmaceutical Industry
Flowserve valves offer several advantages for the pharmaceutical industry.
- Flowserve offers a proven list of sanitary valves and seals for the active pharmaceutical ingredients (API) manufactured.
- Difficult high-temperature applications under vacuum are always a challenge that Flowserve products are designed to meet.
- Flowserve offers years of experience in chemical pharmaceutical applications that involve sanitary processes with high-pressure, vacuum, and high-temperature reactionary applications.
- Flowserve product upgrades meet the stringent operating conditions within an API facility to improve the performance, reliability and sterility of pharmaceutical operations.
Types of Valves Used in Biological and Chemical API Processes
The pharmaceutical API industry is segmented into synthetic chemical API and biological API. The processes differ from the very beginning, when the chemical API process starts with chemical products (raw materials A and B), and the biological API starts with seed cells from a master cell culture. Despite the differences in processing, both chemical and biological API processes employ Flowserve Kammer valves in various process steps.
- Kammer valves are automatic sanitary control valves that meet standards for cleaning in place (CIP) and sanitizing in place (SIP)for both biological and chemical API processes.
Biological API Process
Bio-production is becoming more popular as a way to produce safer and more effective drugs, and it is difficult for pirates to duplicate the process.
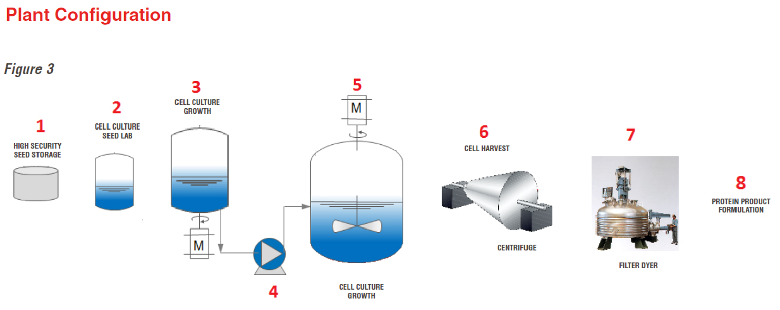
Chemical Pharma API Process
One of the most important features of a high-quality API plant is the flexibility to be able to produce many different fine chemicals in small batches and limit the number of pump and valve changes
The complete production process of API products can be traced in 10 process steps. Since all these products are the base material for a pharmaceutical drug, each production step must be completely controlled per FDA regulations.

Kämmer Valves for Biological and Chemical API Plants
In many of today’s clinical laboratories or biotech pilot plants, valves and related equipment are manually cleaned with a caustic solution, flushed and sterilized in an autoclave after each batch process. This is usually very time-consuming because valves need to be disassembled and cleaned.
This is not a viable option for production plants. Present and future batch sequencing and continuous mode production-scale bioprocessing plants require automatic sanitary control valves that meet standards for cleaning in place (CIP) and sanitizing in place (SIP) designed to drain freely from inlet to outlet. The result is a pure aseptic valve design, free from residue or organisms left behind after cleaning, which can be a source of product contamination.
Aseptic Valves for Biological and Chemical API Plants
Surface finish is of critical importance for the maintenance and cleanliness of the valve, which must meet all the requirements for an aseptic design. It needs to be free of pits and cracks on all wetted parts. Flowserve’s Kämmer® valve series CleanFlow-191000 meets all these requirements.
The Kammer control valve series CleanFlow-191000 has a wide range of applications in biotech, pharmacy and all areas where perfect cleanliness and sterile valves are required. All parts of the easy-maintenance valve that are in contact with the media are made of corrosion-resistant materials, PTFE or silicon. For the aseptic version of this valve series, a PTFE diaphragm seals the media from the environment. CleanFlow-191000 valves:
- have excellent hygienic properties
- are pocket free
- can be cleaned in place
All approvals such as USDA and 3A are fulfilled by these valves. A surface finish of 0.6 Ra for the whole 191000 series is standard. If there are applications that require a higher quality surface finish than 0.6 Ra, Flowserve can easily meet these requirements.
Kammer Valve Specifications
The following Flowserve products handle the majority of control valve applications:
Kämmer CleanFlow-191400
Hygienic control valve, dead pocket-free with integral seat and polished surface finish
- DN 10–DN 100
- 3⁄8–4 in
- PN 16
- CL 150 (max 16 bar)
Kämmer CleanFlow-191700
Aseptic control valve with aseptic diaphragm, dead pocket-free with integral seat and polished surface finish
- DN 10–DN 100
- 3⁄8–4 in
- PN 10
- CL 150 (max 10 bar)
Kammer SmallFlow-385000
Low flow and micro flow control valve for injection applications with highly precise control
- DN 15–DN 25
- ½–1 in
- PN 40–PN 400
- CL 150–CL 2500
Kämmer DrainFlow-051000
Tank bottom outlet valve for tank drain in various configurations
- DN 15–DN 150
- ½–6 in
- PN 10–PN 40
- CL 150–CL 300
Summary
Demand for advanced APIs continues to grow for a variety of reasons:
- the trend toward new high-tech therapeutics
- the emergence of novel and innovative delivery systems
- the evolution of personalized medicines
With expansion of the Chinese pharmaceutical market and increased demand for drugs to treat the aging population worldwide, the manufacture of pharmaceutical APIs will experience continued long-term growth. As the market expands, reliable and long-lasting valves manufactured by Flowserve will be there to support the evolving needs of the pharmaceutical API indust
*All information sourced from Flowserve
